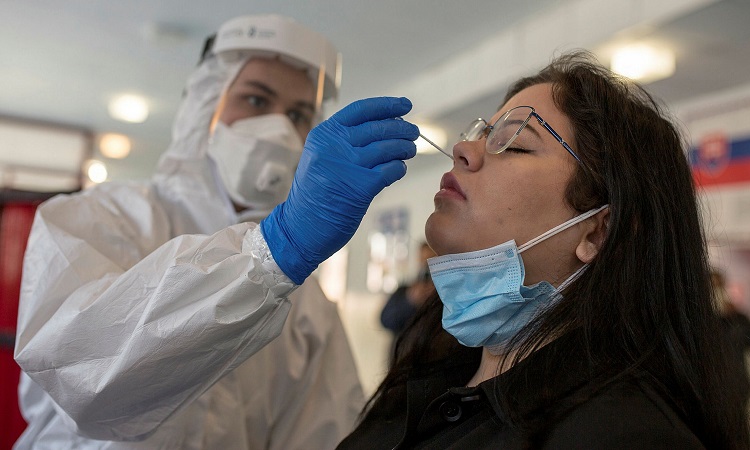
InspectIR COVID-19 analyzes gases to detect chemical compositions associated with SARS-CoV2.
Swabbing, the predominant technique for COVID-19 testing, could be displaced by a more convenient method: breath testing.
The United States Food and Drug Administration (FDA) has granted an emergency permit to InspectIR COVID-19, a test that detects chemical compounds in breath samples to diagnose infections with the SARS-CoV-2 virus.
COVID-19 test with results in less than three minutes
Samples can be collected and tested at doctors’ offices, hospitals or mobile diagnostic sites using the instrument that is about the size of a carry-on bag, the FDA says.
The tests, which have to be carried out by trained and licensed operators, can give results in less than three minutes.
“Today’s authorization is just another example of rapid innovation in diagnostic tests for COVID-19,” said Dr. Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health in a statement.
Pre-approval tests
InspectIR COVID-19 was validated in a large study with 2,409 individuals, including people with and without symptoms.
In the study, a sensitivity of 91.2% was found to identify positive cases and 99.3% to identify negative cases.
The application of this test is ideal in areas with a low incidence of COVID-19 infections, explains the FDA press release.